40. NMR characterization of the diastereomeric composition of a model therapeutic oligonucleotide
B. Mir, M. Gairí, M. T. González, A. Vila-Planas, M. Mayzel, M. Pons, P. Martial,* M. Terrazas* (*Corresponding)
J. Pharm. Sci. 2025, in press. https://doi.org/10.1016/j.xphs.2025.103861
39. NMR characterization of the diastereomeric composition of a model therapeutic oligonucleotide
B. Mir, M. Gairí, M. T. González, A. Vila-Planas, M. Mayzel, M. Pons, P. Martial,* M. Terrazas* (*Corresponding)
chemRxiv 2025, DOI: 10.26434/chemrxiv-2024-vcdxr
38. Systematic study of hybrid triplex topology and stability suggests a general triplex-mediated regulatory mechanism
V. Genna,† G. Portella,† A. Sala,† M. Terrazas,† I. Serrano-Chacón, J. González, N. Villegas, L. Mateo, C. Castellazzi, M. Labrador, A. Aviñó, A. Hospital, A. Gandioso, P. Aloy, I. Brun-Heath, C. González, R. Eritja, M. Orozco (†equal contribution)
Nucleic Acids Res. 2025, 53, gkaf170 | DOI: 10.1093/nar/gkaf170
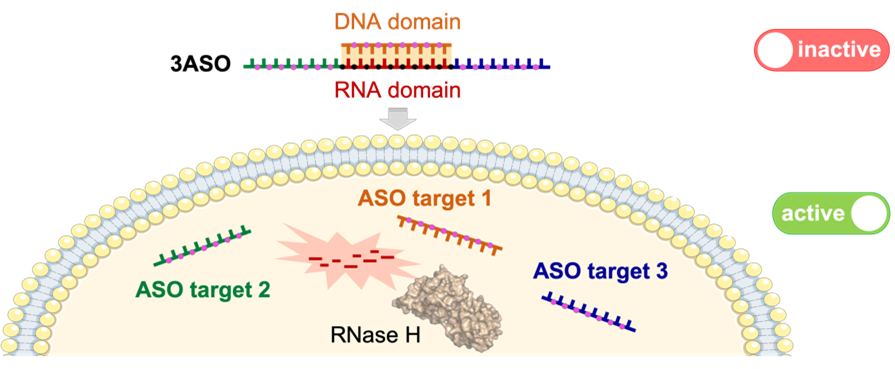
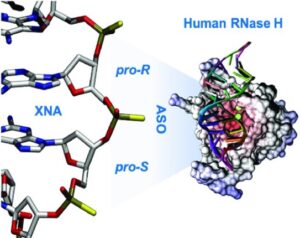
37. RNase H-sensitive multifunctional ASO-based constructs as promising tools for the treatment of multifactorial complex pathologies
Mata-Ventosa, A. Vila-Planas, A. Solsona-Pujol, J. de la Dueña, M. Torrents, E. Izquierdo-García, M. Pastor-Anglada, S. Pérez-Torras,* M. Terrazas* (*Corresponding)
Bioorg. Chem. 2024, 150, 107595 | DOI: 10.1016/j.bioorg.2024.107595
36. Site-specific incorporation of a fluorescent nucleobase analog enhances i-motif stability and allows monitoring of i-motif folding inside cells
B. Mir, I. Serrano-Chacón, P. Medina, V. Macaluso, M. Terrazas, A. Gandioso, M. Garavís, M. Orozco, N. Escaja, C. González
Nucleic Acids Res. 2024, 52, 5575–3389 | DOI: 10.1093/nar/gkae106
35. Special issue “Frontiers in nucleic acid chemistry—in memory of Professor Enrique Pedroso for his outstanding contributions to nucleic acid chemistry
Eritja,* D. Montesarchio,* M. Terrazas* (Co-corresponding)
Molecules 2023, 28 (21), 7278 | DOI: 10.3390/molecules28217278
34. Controlled sulfur-based engineering confers mouldability to phosphorothioate antisense oligonucleotides
Genna, J. Iglesias-Fernández, L. Reyes-Fraile, N. Villegas, K. Guckian, P. Seth, B. Wan, C. Cabrero, M. Terrazas, I. Brun-Heath, C. González, S. Sciabola, A. Villalobos, M. Orozco
Nucleic Acids Res. 2023, 51, 4713–4725 | DOI: 10.1093/nar/gkad309
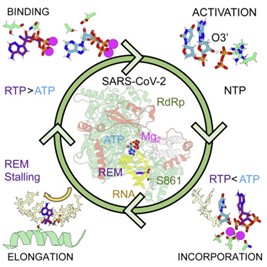
33. Mechanism of reaction of RNA-dependent RNA polymerase from SARS-CoV-2
Juan Aranda, Milosz Wieczór, Montserrat Terrazas, Isabelle Brun-Heath, Modesto Orozco
Chem Catal. 2023, 2, 1084–1099 | DOI: 10.1016/j.checat.2022.03.019
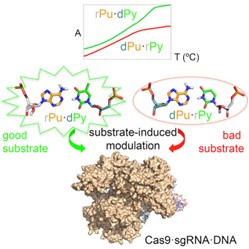
32. Molecular basis of Arginine and Lysine DNA sequence-dependent thermo-stability modulation
Martin, P.D. Dans, M. Wieczór, N. Villegas, I. Brun-Heath, F. Battistini, M. Terrazas, M. Orozco
PLoS Comput. Biol. 2022, 18, e1009749 | DOI: 10.1371/journal.pcbi.1009749
31. The impact of the hydroxymethylcytosine epigenetic signature on DNA structure and function
Battistini, P.D. Dans, M. Terrazas, C.L. Castellazzi, G. Portella, M. Labrador, N. Villegas, I. Brun-Heath, C. González, M. Orozco
PLoS Comput. Biol. 2021, 17, e1009547 | DOI: 10.1371/journal.pcbi.1009547
30. Dynamics-Function Analysis in Catalytic RNA Using NMR Spin Relaxation and Conformationally Restricted Nucleotides
C.G. Hoogstraten, M. Terrazas, A. Aviñó, N.A. White, M. Sumita
In: Scarborough, R.J., Gatignol, A. (eds) Ribozymes. Methods in Molecular Biology, 2021, vol 2167. Humana, New York, NY. | DOI: 10.1007/978-1-0716-0716-9_11
29. The origins and the biological consequences of the Pur/Pyr DNA· RNA asymmetry
Terrazas, V. Genna, G. Portella, N. Villegas, D. Sanchez, C. Arnan, C. Pulido-Quetglas, R. Johnson, R. Guigó, I. Brun-Heath, A. Aviñó, R. Eritja, M. Orozco
CHEM 2019, 5, 1619–1631 | DOI: 10.1016/j.chempr.2019.04.002
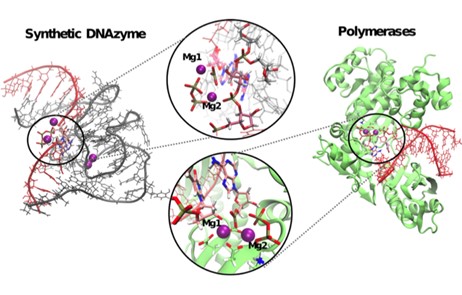
28. An artificial DNAzyme RNA ligase shows a reaction mechanism resembling that of cellular polymerases
Aranda, M. Terrazas, H. Gomez, N. Villegas, M. Orozco
Nat. Catal. 2019, 2, 544–552 | DOI: 10.1038/s41929-019-0290-y
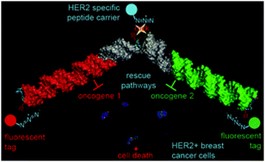
27. A multifunctional toolkit for target-directed cancer therapy
Terrazas,* D. Sánchez, F. Battistini, N. Villegas, I. Brun-Heath, M. Orozco (*Corresponding)
Chem. Commun. 2019, 55, 802–805 | DOI: 10.1039/C8CC08823C
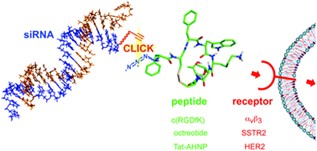
26. Efficient siRNA–peptide conjugation for specific targeted delivery into tumor cells
Gandioso, A. Massaguer, N. Villegas, C. Salvans, D. Sánchez, I. Brun-Heath, V. Marchán,* M. Orozco, M. Terrazas* (*Corresponding)
Chem. Commun. 2017, 53, 2870–2873 | DOI: 10.1039/C6CC10287E
25. Rational design of novel N-alkyl-N capped biostable RNA nanostructures for efficient long-term inhibition of gene expression
Terrazas,* I. Ivani, N. Villegas, C. Paris, C. Salvans, I. Brun-Heath, M. Orozco (* Corresponding)
Nucleic Acids Res. 2016, 44, 4354–4367 | DOI: 10.1093/nar/gkw169
24. Can A denaturant stabilize DNA? Pyridine reverses DNA denaturation in acidic pH
Portella,† M. Terrazas,† N. Villegas, C. González, M. Orozco (†Shared first authoship)
Angew. Chem. Int. Ed. 2015, 54, 10634–10637 | DOI: 10.1002/ange.201503770
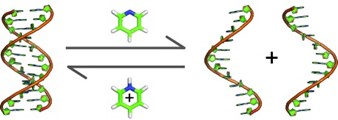
23. Modulation of the RNA interference activity using central mismatched siRNAs and acyclic threoninol nucleic acids (aTNA) units
Alagia, M. Terrazas, R. Eritja
Molecules 2015, 20, 7602–7619 | DOI: 10.3390/molecules20057602
22. RNA/aTNA chimeras: RNAi effects and nucleases resistance of single and double stranded RNAs
A. Alagia, M. Terrazas, R. Eritja
Molecules 2014, 19, 17872–17896 | DOI: 10.3390/molecules191117872
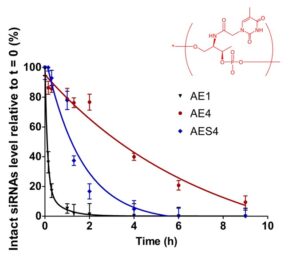
21. Challenges and opportunities for oligonucleotide-based therapeutics by antisense and RNA interference mechanisms
R. Eritja, M. Terrazas, S. Grijalvo, A. Aviñó, A. Alagia, S. Pérez-Rentero, J. Carlos Morales
In: Erdmann, V., Markiewicz, W., Barciszewski, J. (eds) Chemical Biology of Nucleic Acids. RNA Technologies. 2021, Springer, Berlin, Heidelberg. | DOI: 10.1007/978-3-642-54452-1_13
20. Synthesis, RNAi activity and nulcease-resistant properties of apolar carbohydrates siRNA conjugates
Vengut-Climent, M. Terrazas, R. Lucas, M. Arévalo-Ruiz, R. Eritja, J. Carlos Morales
Bioorg. Med. Chem. Lett. 2013, 23, 4048–4051 | DOI: 10.1016/j.bmcl.2013.05.065
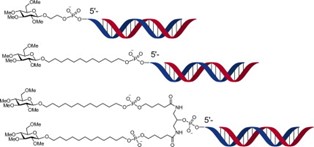
19. Functionalization of the 3′-ends of DNA and RNA strands with N-ethyl-N-coupled nucleosides: A promising approach to avoid 3′-exonuclease-catalyzed hydrolysis of therapeutic oligonucleotides
Terrazas,* A. Alagia, I. Faustino, M. Orozco, R. Eritja* (*Co-corresponding)
ChemBioChem. 2013, 14, 510–520 | DOI: 10.1002/cbic.201200611
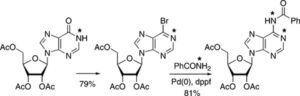
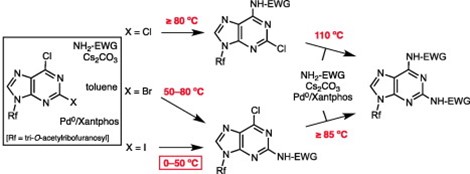
18. Pd-catalysed amidation of 2, 6-dihalopurine nucleosides. Replacement of iodine at 0° C
Bosch, I. Cialîcu, J. Caner, X. Ariza, A.M. Costa, M. Terrazas, J. Vilarrasa
Tetrahedron Lett. 2012, 53, 1358–1362 | DOI: 10.1016/j.tetlet.2012.01.012
17. Synthesis and properties of small interfering RNA duplexes carrying 5-ethyluridine residues
Terrazas, R. Eritja
Mol. Divers. 2011, 15, 677–686 | DOI: 10.1007/s11030-010-9290-1
16. A direct, efficient method for the preparation of siRNAs containing ribo-like North bicyclo[3.1.0]hexane pseudosugars
Terrazas, A. Avino, M.A Siddiqui, V.E. Marquez, R. Eritja
Org. Lett. 2011, 13, 2888–2891 | DOI: 10.1021/ol200909j
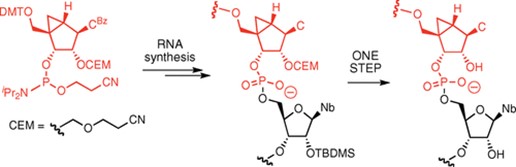
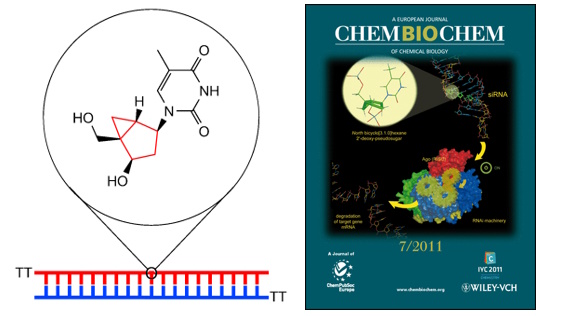
15. Effect of North bicyclo[3.1.0]hexane 2′‐deoxy‐pseudosugars on RNA Interference: A novel class of siRNA modification
Terrazas, S.M. Ocampo, J.C. Perales, V.E. Marquez, R. Eritja
ChemBioChem. 2011, 12, 1056–1065 | DOI: 10.1002/cbic.201000791 – Inside cover picture
14. Synthesis of oligonucleotide–peptide conjugates for biomedical and technological applications
Synthesis of oligonucleotide–peptide conjugates for biomedical and technological applications
A. Aviñó, S. Grijalvo
13. Synthesis and properties of oligonucleotides carrying isoquinoline imidazo[1,2-a]azine fluorescent units
Pérez-Rentero, N. Kielland, M. Terrazas, R. Lavilla, R. Eritja
Bioconj. Chem. 2010, 21, 1622–1628 | DOI: 10.1021/bc1000966
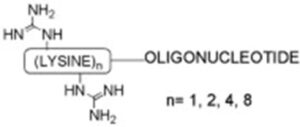
12. Stepwise synthesis of oligonucleotide–peptide conjugates containing guanidinium and lipophilic groups in their 3′-termini
Grijalvo, M. Terrazas, A. Aviñó, R. Eritja
Bioorg. Med. Chem. Lett. 2010, 20, 2144–2147 | DOI: 10.1016/j.bmcl.2010.02.049
11. Modified siRNAs for the study of the PAZ domain
Somoza, M. Terrazas, R. Eritja
Chem. Commun. 2010, 46, 4270–4272 | DOI: 10.1039/C003221B
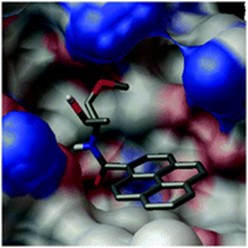
10. RNA major groove modifications improve siRNA stability and biological activity
Terrazas, E.T. Kool
Nucleic Acids Res. 2009, 37, 346–353 | DOI: 10.1093/nar/gkn958
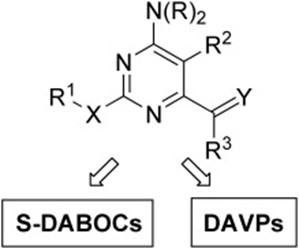
9. A multidisciplinary approach for the identification of novel HIV‐1 non‐nucleoside reverse transcriptase inhibitors: S‐DABOCs and DAVPs
Radi, C. Falciani, L. Contemori, E. Petricci, G. Maga, A. Samuele, S. Zanoli, M. Terrazas, M. Castria, A. Togninelli, J.A. Esté, I. Clotet‐Codina, M. Armand‐Ugón, M. Botta
ChemMedChem 2008, 3, 573–593 | DOI: 10.1002/cmdc.200700198
8. Enzymatically catalyzed DNA synthesis using L‐Asp‐dGMP, L‐Asp‐dCMP, and L‐Asp‐dTMP
Terrazas, P. Marlière, P. Herdewijn
Chem. & Biodivers. 2008, 5, 31–39 | DOI: 10.1002/cbdv.200890013
7. Enzymatically catalyzed DNA synthesis using L‐Asp‐dGMP, L‐Asp‐dCMP, and L‐Asp‐dTMP
Terrazas, P. Marlière, P. Herdewijn
In: Piet Herdewijn, M. Volkan Kısakürek (eds.), Origin of Life. Chemical approach, 2008, Wiley-VCH Verlag GmbH & Co. KGaA, p. 363–371
6. Polymerase-catalyzed synthesis of DNA from phosphoramidate conjugates of deoxynucleotides and amino acids
O. Adelfinskaya, M. Terrazas, M. Froeyen, P. Marliere, K. Nauwelaerts, P. Herdewijn
Nucleic Acids Res. 2007, 35, 5060–5072 | DOI: 10.1093/nar/gkm498
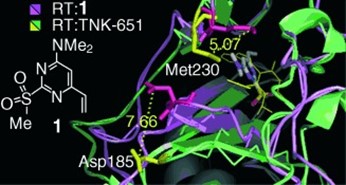
5. Discovery of non-nucleoside inhibitors of HIV-1 reverse transcriptase competing with the nucleotide substrate
G. Maga, M. Radi, S. Zanoli, F. Manetti, R. Cancio, U. Hübscher, S. Spadari, C. Falciani, M. Terrazas, J. Vilarrasa, M. Botta
Angew. Chem. Int. Ed. 2007, 46, 1810–1813 | DOI: 10.1002/anie.200604165
4. Advantages of the Ns group in the reactions of N1-SO2R inosines with benzylamine and with 15NH3
M. Terrazas, X. Ariza, J. Vilarrasa
Tetrahedron Lett. 2005, 31, 5127–5130 | DOI: 10.1016/j.tetlet.2005.05.137
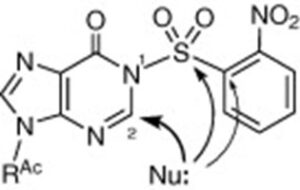
3. [N,1-15N2]-2‘-Deoxyadenosines
M. Terrazas, X. Ariza, J. Vilarrasa
Org. Lett. 2005, 7, 2477–2479 | DOI: 10.1021/ol050788w
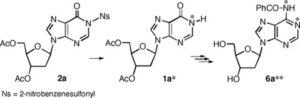
2. A novel nucleophilic approach to 1-alkyladenosines. A two-step synthesis of [1-15N] adenosine from inosine
M. Terrazas, X. Ariza, J. Farràs, J. Vilarrasa
Chem. Commun. 2005, 31, 3968–3970 | DOI: 10.1039/B505183E
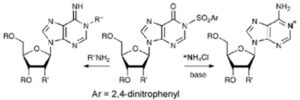
- A direct, efficient method for the preparation of N6-protected 15N-labeled adenosines
M. Terrazas, X. Ariza, J. Farràs, J.M. Guisado-Yang, J. Vilarrasa
J. Org. Chem. 2004, 69, 5473–5475 | DOI: 10.1021/jo049490u